Intercept (ICPT) to Restructure Operations, Cut Workforce
Intercept Pharmaceuticals, Inc. ICPT announced a restructuring program in the wake of the recent complete response letter (CRL) issued by the FDA to its new drug application (NDA) seeking approval for obeticholic acid (OCA) for treating pre-cirrhotic fibrosis due to nonalcoholic steatohepatitis (NASH).
Intercept will discontinue all NASH-related investments and immediately begin the process of closing out the REGENERATE study. Intercept expects to substantially complete the trial shut-down process by the end of 2023.
Apart from closing out REGENERATE, Intercept is also terminating all other NASH-related spending within its R&D, commercial, medical affairs and administrative functions.
The company will also cut one-third of its workforce to reduce operating expenses. Intercept expects to initiate workforce reductions in the third quarter of 2023, with a vast majorty to be completed by the end of 2023.
Nevertheless, Intercept plans to maintain the scale of its current field sales organization to support the growth potential of Ocaliva.
We note that OCA is already approved under the brand name Ocaliva for treating primary biliary cholangitis (in combination with ursodeoxycholic acid [UDCA]) in adults with an inadequate response to UDCA alone or as a monotherapy for adults intolerant to UDCA.
The restructuring program is expected to reduce non-GAAP adjusted operating expenses by approximately $140 million.
Concurrently, Intercept has lowered its non-GAAP adjusted operating expense guidance to $350-$370 million for 2023 (previous guidance: $360-$390 million). The updated guidance includes expenses to wind down the REGENERATE study and stop all other NASH-related activity, as well as estimated charges of approximately $16 million related to the planned workforce reduction in 2023. Intercept expects majority of restructuring costs to be incurred during the third quarter of 2023.
Intercept also reiterated its 2023 Ocaliva sales guidance of $310-$340 million, as compared to sales of $285.7 million in 2022.
Intercept’s shares have lost 3.8% in the year so far compared with the industry’s 8.2% decline.
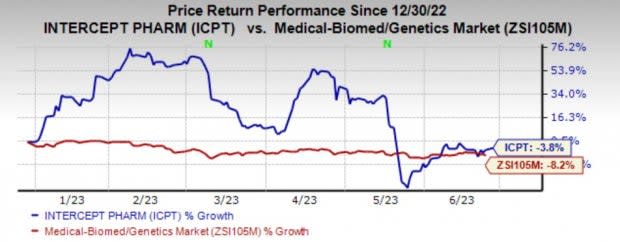
Image Source: Zacks Investment Research
Meanwhile, Intercept will continue to invest in Ocaliva and other pipeline candidates. The company remains on track for a planned regulatory submission to the FDA later this year in support of fulfilling post-marketing requirements for Ocaliva in PBC. This submission will include data from the post-marketing study COBALT and supplementary real-world evidence from large datasets in the United States and Europe.
Intercept will also invest in its fixed-dose combination of OCA and bezafibrate, a peroxisome proliferator-activated receptor agonist. Two phase II studies are currently ongoing to evaluate a range of therapeutic doses for the combination, with planned interim analyses from both studies expected to be completed in 2023. The planned interim analyses from phase II studies, in addition to phase I and preclinical data, will serve as the basis for an end-of-phase II meeting with the FDA.
In addition, Intercept continues to advance the phase IIa FRESH study for INT-787, a next-generation farnesoid X receptor (FXR) agonist, to establish a proof-of-concept in severe alcohol-associated hepatitis.
The CRL was disappointing for Intercept and now it has to look for an alternative growth trajectory. While the development of the pipeline apart from NASH candidates is encouraging, the successful development of OCA for NASH would have been a significant boost for the company. The NASH market holds potential but is quite challenging with no approved therapies.
Several other companies are attempting to develop a treatment for the same condition.
Last month, clinical-stage biopharmaceutical company Viking Therapeutics, Inc. VKTX announced positive top-line results from its phase IIb clinical trial of VK2809 in patients with biopsy-confirmed NASH. The study successfully achieved its primary endpoint, with patients receiving VK2809 experiencing statistically significant reductions in liver fat content from baseline to week 12 as compared with placebo. Additionally, VK2809-treated patients demonstrated statistically significant reductions in low-density lipoprotein cholesterol (LDL-C), triglycerides and atherogenic lipoproteins compared with placebo.
Viking expects to report 52-week biopsy data from the study in the first half of next year.
Intercept currently has a Zacks Rank #3 (Hold). A better-ranked stock in the healthcare sector is Ligand Pharmaceuticals LGND, sporting a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Over the past 60 days, earnings estimates for LGND have increased by $1.09 per share to $5.25. LGND topped earnings estimates in two of the last four quarters and missed in the remaining two, the average surprise being 21.50%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Ligand Pharmaceuticals Incorporated (LGND) : Free Stock Analysis Report
Intercept Pharmaceuticals, Inc. (ICPT) : Free Stock Analysis Report
Viking Therapeutics, Inc. (VKTX) : Free Stock Analysis Report
